Towards robust alkane oxidation catalysts: electronic variations in non-heme iron(ii) complexes and their effect in catalytic alkane oxidation†
Abstract
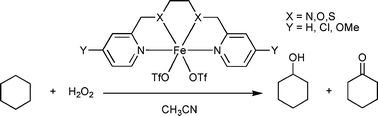
A series of non-heme iron(II) bis(triflate) complexes containing linear and tripodal tetradentate

 Please wait while we load your content...
Please wait while we load your content...