Spectroelectrochemical properties of homo- and heteroleptic ruthenium and osmium binuclear complexes: intercomponent communication as a function of energy differences between HOMO levels of bridge and metal centres†
Abstract
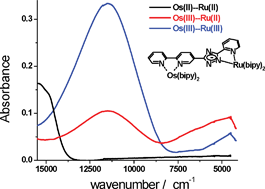
A series of binuclear ruthenium and osmium complexes [(bipy)2Ru(qpy)Ru(bipy)2]4+ (1), [(bipy)2Os(qpy)Os(bipy)2]4+ (2), [(bipy)2Ru(pytr-bipy)Ru(bipy)2]3+ (3), [(bipy)2Ru(pytr-bipy)Os(bipy)2]3+ (4), [(bipy)2Os(pytr-bipy)Ru(bipy)2]3+(5) and [(bipy)2Os(bpbt)Os(bipy)2]2+ (6) {

 Please wait while we load your content...
Please wait while we load your content...