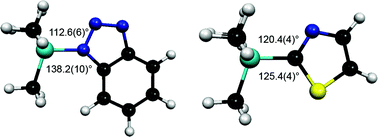
The structures of 1-trimethylsilyl-1,2,3-benzotriazole and 2-trimethylsilyl-1,3-thiazole have been determined by gas electron diffraction and computational methods. While 1-trimethylsilyl-1,2,3-benzotriazole shows a significant asymmetry in the way the SiMe3 groups bonds to the ring system, the same is not true for 2-trimethylsilyl-1,3-thiazole. However, it has been shown that when the positions of formal single and double bonds in the rings systems are considered, the silyl groups in both compounds are displaced towards the neighbouring ring nitrogen atom. Calculated structures of a series of analogous compounds with different substituents on silicon show only minor variations in the extent of the distortion, although with hydrogen instead of a silyl group the displacement is significantly smaller.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...