Insertion of a dimethyl acetylenedicarboxylate (DMAD) into the Ru–C bond in a cycloruthenated complex Ru[OC6H3(2-CH2)(6-Me)-κ2O,C](PMe3)4 (2) has been achieved to give a seven-membered oxaruthenacycle Ru[OC6H3{2-CH2C(CO2Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CO2Me)}(6-Me)-κ2O,C](PMe3)3 (3) in 47% yield. The molecular structure of 3 by X-ray analysis shows an agostic interaction between the ruthenium and one of the benzylic methylene protons. Complex 3 shows fluxional behaviour in solution and the variable temperature NMR studies suggest this fluxionality to be responsible for the turnstile rotation of three PMe3 ligands and the rotation of the α-methoxycarbonyl group. Heating of a toluene solution of 3 at 100 °C for 2 h results in the 1,3-H shift reaction in 3 to give a κ1O,η3-C,C′,C″ allylic complex Ru[OC6H3{2-CHC(CO2Me)CH(CO2Me)}(6-Me)-κ1O,η3C,C′,C″](PMe3)3 (6) (80–90%), whose molecular structure is revealed by X-ray analysis. Acidolyses of 3 and 6 give 2-[(Z)-2′,3′-bis(methoxycarbonyl)allyl]-6-methylphenol (7) (88%) and 2-[(Z)-2′,3′-bis(methoxycarbonyl)propenyl]-6-methylphenol (8) (47%), respectively, and iodolyses of 3 and 6 produce 2,3-bis(methoxycarbonyl)-8-methyl-4H-benzopyran (9) (24%) and 2,3-bis(methoxycarbonyl)-8-methyl-2H-benzopyran (10) (48%), respectively.
C(CO2Me)}(6-Me)-κ2O,C](PMe3)3 (3) in 47% yield. The molecular structure of 3 by X-ray analysis shows an agostic interaction between the ruthenium and one of the benzylic methylene protons. Complex 3 shows fluxional behaviour in solution and the variable temperature NMR studies suggest this fluxionality to be responsible for the turnstile rotation of three PMe3 ligands and the rotation of the α-methoxycarbonyl group. Heating of a toluene solution of 3 at 100 °C for 2 h results in the 1,3-H shift reaction in 3 to give a κ1O,η3-C,C′,C″ allylic complex Ru[OC6H3{2-CHC(CO2Me)CH(CO2Me)}(6-Me)-κ1O,η3C,C′,C″](PMe3)3 (6) (80–90%), whose molecular structure is revealed by X-ray analysis. Acidolyses of 3 and 6 give 2-[(Z)-2′,3′-bis(methoxycarbonyl)allyl]-6-methylphenol (7) (88%) and 2-[(Z)-2′,3′-bis(methoxycarbonyl)propenyl]-6-methylphenol (8) (47%), respectively, and iodolyses of 3 and 6 produce 2,3-bis(methoxycarbonyl)-8-methyl-4H-benzopyran (9) (24%) and 2,3-bis(methoxycarbonyl)-8-methyl-2H-benzopyran (10) (48%), respectively.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CO2Me)}(6-Me)-κ2O,C](PMe3)3 (3) in 47% yield. The molecular structure of 3 by
C(CO2Me)}(6-Me)-κ2O,C](PMe3)3 (3) in 47% yield. The molecular structure of 3 by 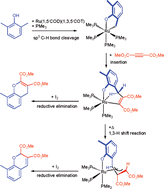

 Please wait while we load your content...
Please wait while we load your content...