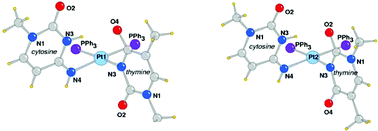
The mixed nucleobases complexes cis-[L2Pt{1-MeTy(-H)}(1-MeCy,N3)]NO3 (L = PPh3, 1a; PMePh2, 1b), containing the N(3)-deprotonated 1-methylthymine (1-MeTy(-H)) and the neutral 1-methylcytosine (1-MeCy) have been prepared and characterised. The compounds were obtained by reacting the hydroxo complexes cis-[L2Pt(μ-OH)]2(NO3)2 with 1-methylthymine (1-MeTy), followed by the addition of 1 equivalent of 1-MeCy. In solution of DMSO, DMF or chlorinated solvents, 1a converts quantitatively into the isomer cis-[L2Pt{1-MeTy(-H)}(1-MeCy,N4)]NO3 (2a) containing the tautomeric form of the cytosine stabilized through the coordination at the N(4) atom, as shown by single-crystal X-ray analysis. The structural determination of 2a shows the presence in the unit cell of two crystallographic independent complexes having similar conformation, with a different orientation of the two nucleobases (head–head and head–tail) according to the presence of both isomers in solution. Complex 1b, having the less hindered PMePh2 ligands, in DMSO solution, contains the tautomeric forms of the cytosine in equilibrium and the migration of the metal from the N(3) to N(4) site occurs only to a minor extent.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...