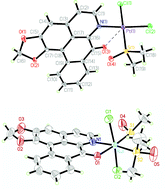
Liriodenine (L), an active component of the anticancer traditional Chinese medicine (TCM), was isolated from Zanthoxylum nitidum. Its reactions with Pt(II) and Ru(II) afforded three metal complexes: cis-[PtCl2(L)] (1), cis-[PtCl2(L)(DMSO)] (2), and cis-[RuCl2(L)(DMSO)2]·1.5H2O (3), the crystal structures of L, 2 and 3 were determined by single-crystal X-ray diffraction methods. These complexes were fully characterized by elemental analysis, IR spectrophotometry, 1H and 13C NMR spectroscopies, and ES mass spectrometry. The in vitro cytotoxicity of L and complexes 1–3 against 11 human tumour cell lines was assayed. The metal-based compounds exhibit enhanced cytotoxicity vs. free L, suggesting that these compounds display synergy in the combination of metal ions and liriodenine. The binding properties of L and its complexes 1–3 to ct-DNA were investigated through UV-vis, fluorescence, CD spectra, viscosity and agarose gels electrophoretic measurements.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...