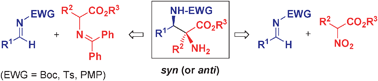
Optically active α,β-diamino acids are very attractive targets in organic synthesis because of their wide-ranging biological significance and high versatility as synthetic building blocks. Efficient synthesis of such non-proteinogenic amino acid derivatives must face the challenge of generating two contiguous stereocenters with complete diastereo- and enantiocontrol in flexible, acyclic molecules. The catalytic asymmetric direct Mannich reaction has provided elegant and efficient solutions for the stereocontrolled assembly of both syn- and anti-α,β-diamino acid derivatives, including those with a α-tetrasubstituted carbon stereocenter, with the aid of either organometallic or purely organic chiral catalysts (or the combination of both). This tutorial review highlights progress in this area, which has recently been boosted through two complementary strategies: the direct Mannich reaction of glycine ester Schiff bases with imines and the direct aza-Henry reaction between nitro compounds and imines.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...