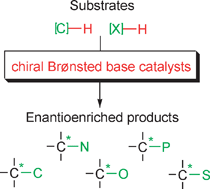
Chiral, metal-free Brønsted bases have been demonstrated capable of catalyzing several types of C–C and C–X bond-forming reactions with high chemical and stereochemical efficiency, thereby complementing other major organocatalytic asymmetric approaches such as enamine catalysis. This critical review describes the most fundamental developments in the area, which have been documented in the last couple of years, along with major mechanistic implications. Discussion includes the limitations of the present protocols, identifies the most salient gaps, and gives a prospect of future improvements and applications (122 references).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...