On the low volatility of cyclic esters: an infrared spectroscopy comparison between dimers of γ-butyrolactone and methyl propionate†
Abstract
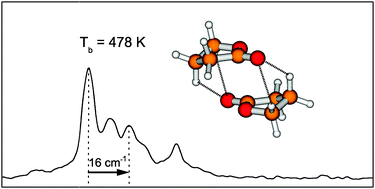
The dramatically lower volatility of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond contacts. The
C hydrogen bond contacts. The ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C dipole moments into an unfavorable near-parallel orientation, thus their higher volatility.
O and C–O–C dipole moments into an unfavorable near-parallel orientation, thus their higher volatility.

 Please wait while we load your content...
Please wait while we load your content...