The interplay of van der Waals and weak chemical forces in the adsorption of salicylic acid on NaCl(001)
Abstract
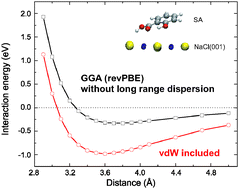
The role of long range dispersion forces plays a significant role in the adsorption of weakly chemisorbed molecules, as demonstrated by our first-principles calculations with the van der Waals density functional (vdW-DF) applied to the model system

 Please wait while we load your content...
Please wait while we load your content...