Dynamic nuclear polarization of water by a nitroxide radical: rigorous treatment of the electron spin saturation and comparison with experiments at 9.2 Tesla
Abstract
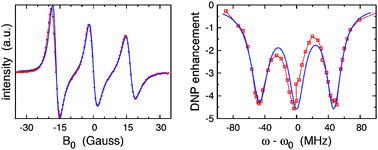
The interaction between nuclear and electronic spins is of interest for structural characterization of biomolecules and biomedical imaging based on nuclear magnetic resonance. The polarization of the nuclear spins can be increased significantly if the electron spin polarization is kept out of equilibrium. We employ semiclassical relaxation theory to analyze the electronic polarization of the two-spin system characteristic of nitroxide radicals. Atomistic molecular dynamics simulations of the nitroxide TEMPOL in
- This article is part of the themed collection: Modern EPR spectroscopy: beyond the EPR spectrum

 Please wait while we load your content...
Please wait while we load your content...