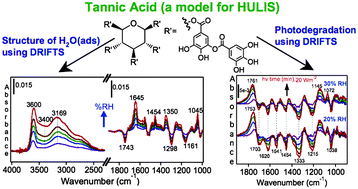
Humic like substances (HULIS) are important components of atmospheric aerosols, yet little is known about their photochemical transformation and the role of adsorbed water in this photochemistry. We report herein in situ and surface-sensitive spectroscopic studies on (1) the photodegradation of solid tannic acid, (2) structure of adsorbed water before and after photodegradation, and (3) the change in the hydrophilicity of tannic acid as a result of this photochemistry. Tannic acid (TA) was chosen as a synthetic proxy for HULIS because it has a defined molecular structure. Photochemical studies were conducted using diffuse reflectance infrared spectroscopy (DRIFTS) as a function of time (3 h), relative humidity (5–30%) and total irradiance (7, 20, 290 W m−2 at 555 nm). Water adsorption isotherm measurements were recorded before and after photodegradation, which provided information on the structure of interfacial water and the thermodynamics of adsorption. The structure of water adsorbed on TA resembles that of water at the interface with polar organic solvents. Difference spectral data collected during irradiation shows loss features in the 1700–1000 cm−1 range and growth in carbonyl features that are blue shifted relative to the starting material, suggesting oxidative photodegradation of TA and formation of aryl aldehydes. Under our experimental conditions, we observed no enhancement in water uptake after photodegradation relative to that on unirradiated samples. The implications of our results to the understanding of heterogeneous photochemistry of HULIS and the role of adsorbed water in these reactions are discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...