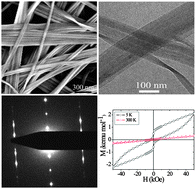
Here we present the synthesis of layered, ferrimagnetic, Co-based organic–inorganic hybrid nanobelts formed through self-organization in a non-aqueous process. The belts are formed solvothermally by reacting cobalt(III) acetylacetonate with benzoic acid in benzyl alcohol at 150 °C. Benzoic acid not only acts as an intercalation molecule for the formation of the layered organic–inorganic hybrid structure, but also as a shape-directing agent for manipulating the product morphology. These nanobelts are typically obtained with a width of 50–200 nm, thickness of 10–20 nm and length of several hundreds of microns. Furthermore, magnetic measurements show that the resulting layered nanobelts exhibit a ferrimagnetic transition at 40 K and superparamagnetic behavior below 40 K. Moreover, the upturn of the magnetization at 5 K and H = 40 kOe indicate a smooth field-induced magnetic transition with a critical field of approximately 4 T.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...