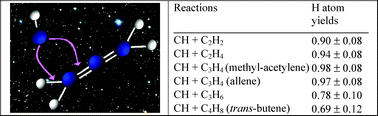
The reactions of the CH radical with several unsaturated hydrocarbons C2H2 (acetylene), C2H4 (ethylene), C3H4 (methyl-acetylene and allene), C3H6 (propene) and C4H8 (trans-butene) were studied at room temperature, in a low-pressure fast-flow reactor. CH(X2Π, v = 0) radicals were obtained from the reaction of CHBr3 with potassium atoms. The overall rate constants at 300 K are CH + C2H2: (3.6 ± 0.6) × 10−10, CH + C2H4: (3.1 ± 0.6) × 10−10, CH + C3H4 (methyl-acetylene): (3.4 ± 0.6) × 10−10, CH + C3H4 (allene): (3.6 ± 0.6) × 10−10, CH + C3H6 (propene): (4.2 ± 0.8) × 10−10 and CH + C4H8 (trans-butene): (4.0 ± 0.80) × 10−10 cm3 molecule−1 s−1 (errors are cited at the level of ±1σ). Absolute atomic hydrogen production was determined by vacuum ultra-violet (VUV) resonance fluorescence, H production from the CH + CH4 reaction being used as a reference. Observed H branching ratios for these CH reactions were: C2H2: 0.90 ± 0.08, C2H4: 0.94 ± 0.08, C3H4 (methyl-acetylene): 0.98 ± 0.08, C3H4 (allene): 0.97 ± 0.08, C3H6 (propene): 0.78 ± 0.10, C4H8 (trans-butene): 0.69 ± 0.12 (errors are cited at the level of ±1σ). A compilation of the available kinetic data on these reactions has been made in order to propose rate coefficients for each possible channel of the different reactions for astrochemical models.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...