A potential of mean force estimator based on nonequilibrium work exponential averages
Abstract
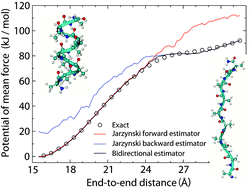
In this article we present a potential of mean force estimator based on measurements of the work performed on a system during out of equilibrium realizations of a process. More specifically, the quantities involved in the estimator are the work exponential averages related to the forward and backward directions of the process and the free energy difference between the end states. Such free energy difference can be estimated without resorting to additional methodologies or data, but exploiting the available work measurements in the Bennett acceptance ratio method. Despite the fact that work exponential averages give strongly biased free energy profiles, a simple combination of them, supplied with an accurate estimate of the free energy difference between the end states, provides good free energies, even for fast pulling velocities of the control parameter. Numerical tests have been performed on a deterministic non-Hamiltonian dynamic system (the folding/unfolding process of one

 Please wait while we load your content...
Please wait while we load your content...