A detailed comparison of centrifugal sudden and J-shift estimates of the reactive properties of the N + N2 reaction
Abstract
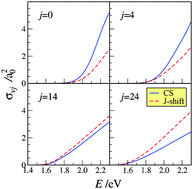
An extended comparison of the reactive properties of the N + N2 exchange reaction calculated on a non-collinear dominant potential energy surface using both a centrifugal sudden and a J-shift quantum method is reported. The choice of carrying out such an investigation for N + N2 is motivated by the fact that the best available (and currently used for spacecraft re-entry simulations) computed set of kinetic data has been worked out using the low level J-shift approximation though based on exact quantum zero total angular momentum probabilities. The fact that our investigation is carried out for a heavy system and a potential energy surface free of wells in the strong interaction region minimizes the occurrence of tunnel, resonance and interference effects which would make the rationalization of the result difficult and the centrifugal sudden treatment less accurate. The study has provided evidence of two important limits of the J-shift approximation: the wrong determination of the maximum value of the total angular momentum quantum number J contributing to reactivity and the lack of deformation of the partial reactive probability dependence on energy at fixed J value. Accordingly, it has been found that the J-shift state-specific cross sections underestimate the corresponding CS values when the initial diatomic rotational energy is low while the situation reverses when the initial diatomic rotational energy is high.

 Please wait while we load your content...
Please wait while we load your content...