Reversible formation of gold nanoparticle–surfactant composite assemblies for the preparation of concentrated colloidal solutions
Abstract
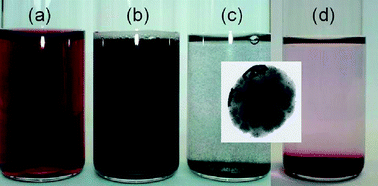
We have developed a simple method for the preparation of nearly mono-dispersed stable gold colloids with a fairly high concentration using a two step procedure. First we synthesize citrate capped

 Please wait while we load your content...
Please wait while we load your content...