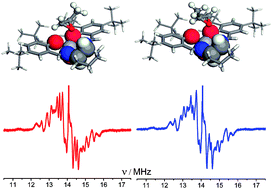
The mode of chiral interaction between a series of asymmetric epoxides (propylene oxide, butylene oxide, epifluorohydrin and epichlorohydrin) and a chiral vanadyl salen complex, N, N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexane-diamino-vanadium (IV) oxide, [VO(1)], was investigated by a range of electron magnetic resonance techniques (EPR, ENDOR, HYSCORE) and DFT. Enantiomer discrimination of the weakly bound epoxides by the vanadyl complex was evident by cw-ENDOR. The origin of this discrimination was attributed to a number of factors including H-bonds, steric properties and electrostatic contributions, which collectively control the outcome of the chiral interaction. DFT revealed the role of a key H-bond, formed between the epoxide oxygen atom (Oepoxide) and the methine proton (Hexo) attached to the asymmetric carbon atom of the chiral vanadyl salen complex, thereby providing a direct pathway for stereochemical communication between complex and substrate. These findings reveal the potential importance of weak outer sphere interactions in stereoselectivities of enantioselective homogeneous catalysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...