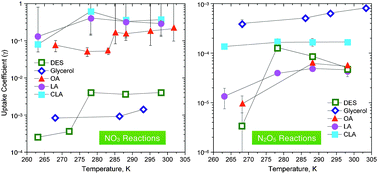
Heterogeneous reactions between NO3 and N2O5 and diethyl sebacate (DES), glycerol, oleic acid (OA), linoleic acid (LA), and conjugated linoleic acid (CLA) were studied to understand better nighttime aerosol chemistry. The reactive uptake coefficient of NO3 on the liquid alkenoic acids (OA, LA, and CLA) was found to be >0.07, which is higher than previous results for unsaturated organics, including alkenoic acids. This reaction could potentially be an important loss process of particle-phase unsaturated organic compounds in the atmosphere and in laboratory secondary organic aerosol studies. The reactive uptake coefficient of N2O5 on liquid glycerol was also found to be relatively large with a value of (3.2–8.5) × 10−4, suggesting that N2O5 heterogeneous reactions with alcohols may also be atmospherically relevant. For all measurements with OA, CLA, and DES, the reactive uptake coefficients decreased significantly upon freezing. One possible explanation is that the liquid reaction is due to both a surface reaction and a bulk reaction and that the freezing process significantly decreases the importance of any bulk reactions. NO3 reactive uptake coefficients for liquid-phase compounds decreased in magnitude in the order: alkenoic acids > DES > glycerol. This is different compared to previous gas-phase studies and the difference may be due to the large viscosity of glycerol compared to the other organic compounds studied. N2O5 reactive uptake coefficients for liquid-phase compounds decreased in magnitude in the order: glycerol > LA > DES ≅ OA ≅ CLA.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...