Chemical and protein shifts in the spectrum of the photoactive yellow protein: a time-dependent density functional theory/molecular mechanics study
Abstract
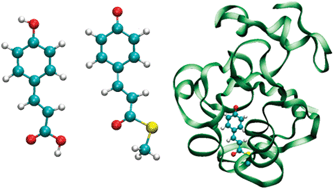
We have studied the light absorption properties of the p-coumaric acid
- This article is part of the themed collection: Time-dependent density-functional theory

 Please wait while we load your content...
Please wait while we load your content...