On the vibronic level structure in the NO3 radical
Part III.† Observation of intensity borrowing via ground state mixing
Abstract
The Ã2E″← ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′2
2A′2 ![[B with combining tilde]](https://www.rsc.org/images/entities/char_0042_0303.gif) 2E′←
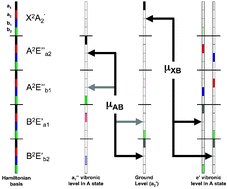
2E′← ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 2A′2 electronic transition, those to a″1 levels involve only negligible upper-state borrowing effects. Rather, it is the vibronic mixing of the ground vibronic level of NO3 with vibrational levels in the
2A′2 electronic transition, those to a″1 levels involve only negligible upper-state borrowing effects. Rather, it is the vibronic mixing of the ground vibronic level of NO3 with vibrational levels in the ![[B with combining tilde]](https://www.rsc.org/images/entities/char_0042_0303.gif) 2E′ electronic state that permit the a″1 levels to be seen in the spectrum. These ideas are supported by vibronic coupling calculations. The fact that the intensities of features corresponding to the two different vibronic symmetries are comparable is thus accidental.
2E′ electronic state that permit the a″1 levels to be seen in the spectrum. These ideas are supported by vibronic coupling calculations. The fact that the intensities of features corresponding to the two different vibronic symmetries are comparable is thus accidental.
- This article is part of the themed collection: Electronic structures and reaction dynamics of open-shell species

 Please wait while we load your content...
Please wait while we load your content...