Oxygen incorporation into strontium titanate single crystals from CO2 dissociation
Abstract
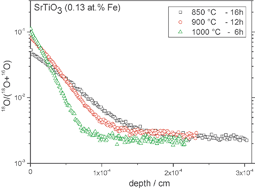
Oxygen incorporation from CO2 into Fe-doped SrTiO3(100) single crystals (0.013 at% Fe, 0.039 at% Fe and 0.13 at% Fe) was investigated. Oxygen incorporation processes using 13C18O2 as the gas source were studied by isotope exchange depth profiling (IEDP) and subsequent secondary-ion
- This article is part of the themed collection: Physical chemistry of solids - The science behind materials engineering

 Please wait while we load your content...
Please wait while we load your content...