In situ investigation of coloration processes in LiNbO3 : MgO during reducing/oxidizing high-temperature treatments
Abstract
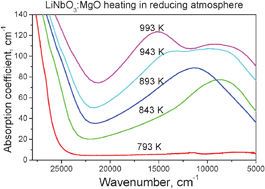
The work presents experimental results of an in situ investigation of optical absorption of LiNbO3 : MgO during reducing (95%Ar + 5%H2) and oxidizing (O2) high-temperature treatments in the temperature range from room temperature to 1073 K. The
- This article is part of the themed collection: Physical chemistry of solids - The science behind materials engineering

 Please wait while we load your content...
Please wait while we load your content...