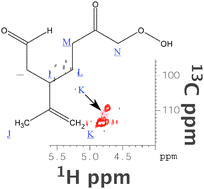
Because it is doubly unsaturated, gaseous limonene and ozone reactions exhibit considerable potential for not only a large quantity of secondary organic aerosol (SOA), but also a diverse and complicated product mixture influenced by reactant conditions. We explore the chemistry of limonene ozonolysis and provide evidence that under low-NOx conditions, the endocyclic double bond is oxidized in the gas phase, while under excess ozone conditions, the residual exocyclic double bond in condensed-phase products is heterogeneously oxidized by ozone. We use regular and multinuclear-multidimensional NMR spectroscopy, in particular 1D 1H-NMR, 1H,1H-COSY (correlation spectroscopy, and 1H,13C-HSQC (heteronuclear single quantum coherence). For structural assignments we simulate 1H and 13C NMR spectra with ACDLabs online, relying representative products consistent with the postulated reaction mechanism. The 1-D 1H-NMR data allow us to quantify the extent to which the residual unsaturation is oxidized with rising ozone, confirming our hypothesis that the residual unsaturation is oxidized via heterogeneous uptake of ozone to fresh SOA particles.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...