Tunability of electronic band gaps from semiconducting to metallic states via tailoring Zn ions in MOFs with Co ions†
Abstract
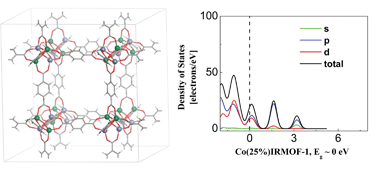
Metal–organic frameworks (MOFs) have recently received much attention as promising candidates for gas storage, chemical separation, and heterogeneous catalysis. However, the applicability of MOFs remains limited due to their relatively large band gaps. Here, on the basis of first-principles theory study, it is demonstrated that this problem could be overcome by tailoring Zn2+ ions in MOFs with Co2+ ions while maintaining the same organic linkers. Density of states and molecular orbitals for MOFs with two elements, Zn and Co ions, show that band gaps ranging from semiconducting to metallic states can be obtained by tailoring the overlaps between the Co and Zn d-orbitals and the O and C p-orbitals.

 Please wait while we load your content...
Please wait while we load your content...