Supramolecular assemblies prepared from an iron(II) tripodal complex, tetrafluoroborate, and alkali metal cations. The effect of cation size on coordination number, anion disorder and hydrogen bonding†
Abstract
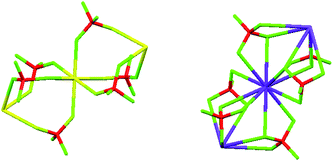
The iron(II) complex cation, 12+, of the 1 : 3 Schiff base condensate of tris(2-aminoethyl)amine (tren) with imidazole-2-carboxaldehyde (H3L1) was reacted with MBF4 (M = Na, K, Rb, Cs and NH4). The products were double salts of the formula {[FeH3L1](BF4)2}·MBF4, 1[M(BF4)3]. The complexes have been characterized by EA, IR, X-ray crystallography, and Mössbauer spectroscopy. The resulting complexes crystallize in P-3 and the cations are located at the origin (0, 0, 0) and c/2 (0, 0, 0.5) on the c axis. Six tetrafluoroborate anions surround the cation sites and each binds to the sodium in a monodentate fashion and to the other cations in a bidentate fashion resulting in distorted octahedral and icosahedral complexes, respectively. Disorder is observed in the tetrafluoroborate anion of both 1[Na(BF4)3] and 1[Cs(BF4)3], resulting in two sets of fluorine atom positions (F set A and F set B), but is absent in the ammonium, potassium, and rubidium double salts. The disorder is attributed to coordination number preference and poor size match, respectively. The tetrafluoroborate anions are involved in extensive hydrogen bonding with the imidazole NH and imine CH of the iron complex. The hydrogen bonding arrangements observed with potassium, rubidium, cesium (F set A) and ammonium are almost identical with one another, but are altered in 1[Na(BF4)3] and 1[Cs(BF4)3] (F set B) complexes. These effects are explained on the basis of cation size. The formation of the double salt results in a shift in the spin equilibrium of the parent iron(II) complex from 62% high spin: 38% low spin at 295 K to pure low spin in the double salts.

 Please wait while we load your content...
Please wait while we load your content...