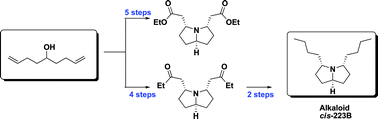
Combining two-directional synthesis and tandem reactions: new access to 3,5-disubstituted pyrrolizidines and first total synthesis of alkaloidcis-223B†
Abstract
Two new tandem reactions for the synthesis of 3,5-disubstituted pyrrolizidines and the first total synthesis of

 Please wait while we load your content...
Please wait while we load your content...