Unusual reactivity of lanthanide borohydride complexes leading to a borane complex†
Abstract
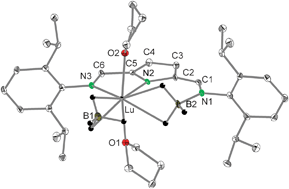
Depending on the ionic radius of the central metal atom the BH4−group, which usually reacts in lanthanide chemistry like a pseudo halide, can be involved in redox chemistry and the resulting product contains an N–BH3 unit, which binds in an unusual η2-fashion onto the metal atom.

 Please wait while we load your content...
Please wait while we load your content...