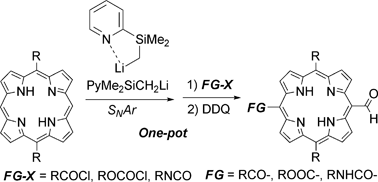
An efficient one-pot procedure for asymmetric bifunctionalization of 5,15-disubstituted porphyrins: a simple preparation of mesoacyl-, alkoxycarbonyl-, and carbamoyl-substituted meso-formylporphyrins†
Abstract
An efficient one-pot procedure which converts 5,15-disubstituted

 Please wait while we load your content...
Please wait while we load your content...