Unprecedented carbon–carbon bond cleavage in nucleophilic aziridine ring opening reaction, efficient ring transformation of aziridines to imidazolidin-4-ones†
Abstract
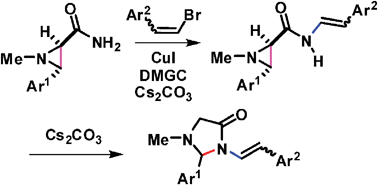
N-Styryl-3-aryl-1-methylaziridine-2-carboxamides, which were readily obtained from the cross coupling reaction between 3-aryl-1-methylaziridine-2-carboxamides and 1-aryl-2-bromoethenes catalyzed by CuI/

 Please wait while we load your content...
Please wait while we load your content...