Fluorescence detection of mercury ions in aqueous media with the complex of a cationic oligopyrene derivative and oligothymine
Abstract
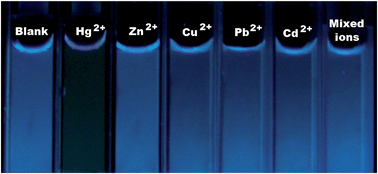
A water-soluble complex of a cationic oligopyrene derivative, oligo(N1,N1,N1,N4,N4,N4-hexamethyl-2-(4-(pyren-1-yl) butanoyloxy) butane-1,4-diaminium bromide), and oligothymine has been prepared. The fluorescence sensor based on this complex showed high sensitivity and selectivity toward mercury(II) ions in aqueous media. The transduction mechanism of this sensor is based on the fluorescence self-quenching of oligopyrene in watervia the interchain π-stacking arising from the specific thymine–Hg–thymine complexation. The detection limit of this sensor was measured to be as low as 5 × 10−9 mol L−1. In addition, the fluorescent color change of the complex solution allows naked-eye detection of mercury(II) ions with an extremely low detection limit of 5 × 10−6 mol L−1.

 Please wait while we load your content...
Please wait while we load your content...