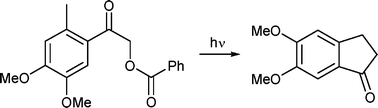
A photoenolization reaction is shown to be the key reaction step in the preparation of substituted indan-1-ones as convenient precursors for the synthesis of donepezil, a well-known acetylcholinesterase inhibitor. Model 2,5-dialkylphenacyl chlorides, differently substituted in the α-carbon position, were found to produce indan-1-ones upon irradiation in non-nucleophilic solvents in high chemical yields via hydrogen chloride release. While direct excitation of 4,5-dimethoxy-2-methylphenacyl chloride led to a complex mixture of photoproducts, photolysis of the corresponding benzoate was found to form 5,6-dimethoxyindan-1-one in 62–72% chemical yields and a relatively low quantum efficiency (Φ ∼ 0.02). This compound can then be easily converted to donepezil by standard synthetic steps described in the literature. Isotopic exchange and quenching experiments revealed that the product is obtained by the photoenolization process via the triplet excited state, while minor side-photoproducts originate from the singlet excited state. Irradiation of the reactant in neat acetone, used both as a triplet sensitizer and solvent at the same time, was found to form 5,6-dimethoxyindan-1-one exclusively in high (90%) chemical yield.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...