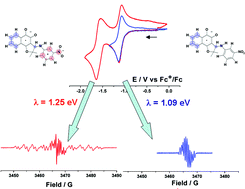
In this work, the electrochemical behaviour of an antitumoral nitro o-quinone derivative obtained from 3-bromo-nor-β-lapachone was studied. Cyclic voltammetric experiments, in acetonitrile solution, revealed that both quinone and nitro functions are reduced independently as quasi-reversible one-electron transfer processes in this order. Depending on the reduction potential, a radical anion or a biradical dianion is obtained. The formation of these paramagnetic species was confirmed by performing in situ Electrochemical-Electron Spin Resonance (E-ESR) experiments. Analysis of the kinetics of electron transfer associated to those electron uptake processes, in terms of the Marcus–Hush–Levich model, revealed differences in the reorganization energy (λ(k)) for both steps (λ(I): 1.07–1.11 eV; λ(II): 1.21–1.30 eV). By evaluating the conformations of the radical and biradical systems by calculations at the BLYP//TZVP level of theory, it was found that the inner component, for the second reduction process (λ(II)) was approximately 72% of λ(II), reflecting modifications in the molecular structure during the radical anion–biradical dianion transition. This change is also reflected in the differences presented by line widths of the ESR signals of both electrogenerated radical and diradical species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...