On the effect of donor and acceptor substituents on the behaviour of light-driven rotary molecular motors
Abstract
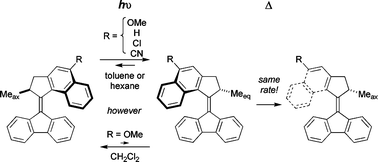
Light-driven rotary molecular motors based on overcrowded alkenes can be substituted with electron-donating and electron-withdrawing substituents (R = OMe, Cl and CN) in direct conjugation with the central double bond (the axis of rotation) without having a significant influence on the rate-limiting, thermal isomerisation step of their rotary cycle. This indicates that in this system, it is predominantly steric factors that determine the barrier to the thermal helix inversion. In contrast, the quantum yield and photoequilibria in the photochemical step were found to be quite sensitive to the combination of substituent and solvent employed.

 Please wait while we load your content...
Please wait while we load your content...