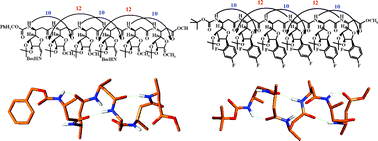
New C-linked carbo-β-amino acids (β-Caas), Cbz-(S)-β-Caa-(NHBoc)-OMe (1) and Cbz-(R)-β-Caa-(NHBoc)-OMe (2), with an additional amine group (methylamino group of NHBoc) at the C-1 position of the lyxofuranoside side chain and Boc-(S)-β-Caa-(diFP)-OMe (3) and Boc-(R)-β-Caa-(diFP)-OMe (4), with a C-difluorophenyl (diFP) moiety at the anomeric position of the lyxofuranoside side chain were prepared from D-mannose. β-Peptides [tetra- and hexapeptides] were synthesized from these β-Caas, ‘epimeric’ [at the amine stereocentre (Cβ)], using the concept of ‘alternating chirality’ to carry out their conformational studies [NMR (CDCl3), CD and MD]. In the monomer design, it was envisaged that the presence of an additional amine group in 1 or 2 would help in solubilizing the peptides in water, while, the C-difluorophenyl (diFP) moiety of 3 and 4 is expected to enhance the biological activity. The peptides having 1 and 2, though could not retain their 12–10-mixed helices in water, have shown moderate activity against Gram positive and Gram negative bacterial strains. The peptides prepared from 3 and 4, much against our expectations, did not display any biological activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...