High physiological thermal triplex stability optimization of twisted intercalating nucleic acids (TINA)†
Abstract
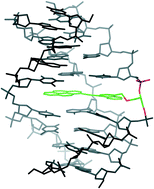
The structure of the monomer (R)-1-O-[4-(1-pyrenylethynyl)phenylmethyl]glycerol (1) in twisted intercalating nucleic acids (TINA) was optimized for stabilizing interactions between the

 Please wait while we load your content...
Please wait while we load your content...