First total synthesis of (R,R,R)- and (3R,5S,9R)-bejarol by gold-catalyzed allene cycloisomerization and determination of absolute configuration of the natural product
Abstract
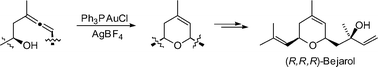
The first total synthesis of (R,R,R)-bejarol and its (3R,5S,9R)-isomer has been accomplished which confirms the absolute configuration of the natural products. The key step is the gold-catalyzed cycloisomerization of the enantiomerically pure β-hydroxyallenes 12/13 to the corresponding dihydropyrans 14/15.

 Please wait while we load your content...
Please wait while we load your content...