Novel photosystem involving protonation and deprotonation processes modelled on a PYP photocycle†
Abstract
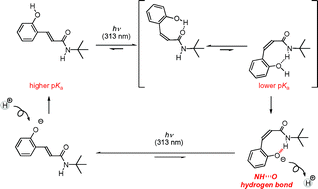
The synthesis of novel ortho-coumaric acid derivatives, with an amide group linked with an olefin moiety, which introduced photoinduced switching of the intramolecular hydrogen bonds is presented. An intramolecular OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond formed in a Z-phenol compound was switched to an intramolecular NH⋯O hydrogen bond in Zphenolate state via deprotonation. The pKa value of the Z-phenol derivative was lower than that of
C hydrogen bond formed in a Z-phenol compound was switched to an intramolecular NH⋯O hydrogen bond in Zphenolate state via deprotonation. The pKa value of the Z-phenol derivative was lower than that of

 Please wait while we load your content...
Please wait while we load your content...