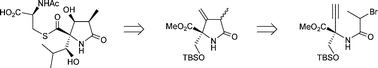
Treatment of the acetylenic bromoamide 42c, derived from the enantiopure α-amino alcohol 40, with Bu3SnH–AlBN results in an efficient 5-exo dig radical cyclisation to the 4-methylenepyrrolidinone 43/44 (2 : 1). Cleavage of the alkene bond in 43/44, using O3–Me2S, next gave the corresponding 4-ketopyrrolidinone 45/46. α-Phenylsulfanylation of 45/46, using S-methyl-p-toluenethiosulfonate–Et3N, proceeded in a stereoselective manner and led to the methylsulfanyl derivative 48 (ca. 9 : 1 selectivity). Manipulation of the functionality in 48, using two separate sequences, then led to the substituted pyrrolidinones 49b, 50 and 53 which are advanced intermediates in a previous synthesis of (+)-lactacystin 1. In related studies, the acetylenic bromoamide 28a containing all the carbon atoms in lactacystin was synthesised, but this substrate failed to undergo an anticipated radical cyclisation to the 4-methylenepyrrolidinone 30, analogous to 43/44. Instead, only the product of reduction of 28a, i.e.28b, was produced, possibly resulting from adventitious intramolecular hydrogen-abstraction processes from the carbon centred radical intermediate 29, i.e.32 to 33 and/or 31 to 34.

 Please wait while we load your content...
Please wait while we load your content...