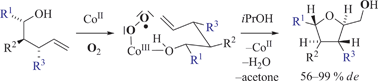
Bishomoallylic alcohols (pent-4-en-1-ols) underwent efficient oxidative cyclizations, if treated with O2 and bis{2,2,2-trifluoromethyl-1-[(1R,4S)-1,7,7-trimethyl-2-(oxo-κO)bicyclo[2.2.1]hept-3-yliden]ethanolato-κO}cobalt(II) in solutions of 2-propanol at 60 °C. Ring closures occurred diastereoselectively and afforded 2,3-trans- (96% de), 2,4-cis- (∼60% de), and 2,5-trans-substituted (>99% de) (phenyl)tetrahydrofur-2-ylmethanols as major components. Formation of bicyclic compounds and a 2,3,4,5-substituted oxolane was feasible as exemplified by syntheses of oxabicyclo[4.3.0]nonylmethanols and a derivative of natural product magnosalicin in 61–72% (90–99% de). The effectiveness of tetrahydrofuran synthesis was critically dependent on (i) solvent, (ii) reaction temperature, (iii) initial cobalt concentration, (iv) chain length between hydroxyl and vinyl groups, and (v) substitution at reacting entities. A sequence is proposed for rationalizing observed selectivities.

 Please wait while we load your content...
Please wait while we load your content...