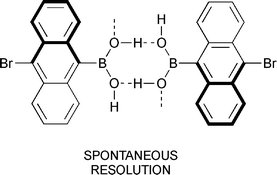
The solid state structures of a number of ortho-substituted arylboronic acids, ortho-bromophenyl, ortho-phenylphenyl, pentamethylphenyl, and 10-bromo-9-anthryl, were determined by X-ray diffraction techniques. All boronic acids investigated form dimers in the solid state, but the interconnection of dimers to ribbons differs from that of the parent phenylboronic acid. Pentamethylphenylboronic acid only uses onehydrogen bond but an additional OH–π interaction for connection of dimers, while all others investigated employ two hydrogen bonds for interconnection of dimers to ribbons. 10-Bromo-9-anthrylboronic acid is found to undergo spontaneous resolution of its enantiomers to a racemic conglomerate upon crystallization.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...