Aryl triflates: useful coupling partners for the direct arylation of heteroaryl derivatives via Pd-catalyzed C–H activation–functionalization†
Abstract
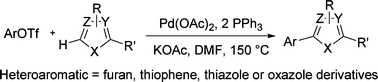
Aryl triflates were found to be suitable partners for the palladium catalyzed direct arylation of heteroaromatics via C–H activation–functionalization. The reaction conditions and the catalyst have a determining influence on the yields. The system combining PPh3 and Pd(OAc)2 using

 Please wait while we load your content...
Please wait while we load your content...