Pyrene derived functionalized low molecular weight organic gelators and gels†
Abstract
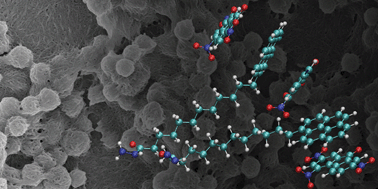
Functional groups were observed to disturb gel formation strongly, if they interact with each other within the same supramolecule due to the formation of competitive structures. Preventing such interactions restored the original gel properties. A gel with weaker supramolecular bonding than the binding between the functional groups was successfully made by separating the functional groups by distance. The π–π-interaction was found to be of negligible significance to the supramolecular binding energy, but probably essential to align the molecules to a one-dimensional chain and bring them into the range of van der Waals forces mainly responsible for the binding in this system.
All molecules were characterized by spectroscopic techniques and
- This article is part of the themed collection: Bio-inspired materials

 Please wait while we load your content...
Please wait while we load your content...