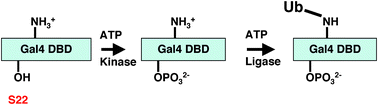
Recent analysis of a Gal4 mutant (Gap71) carrying three point mutations (S22D, K23Q and K25F) in its DNA-binding domain (DBD), has demonstrated that it cannot occupy GAL promoters efficiently in cells and that it is not mono-ubiquitylated, suggesting a functional link between this modification and stable DNA binding in cells. The mechanistic underpinning of this phenotype is that this protein is hypersensitive to a newly discovered activity of the proteasomal ATPases—their ability to actively dissociate transcription factor-DNA complexes after direct interaction with the activation domain. In this paper, we examine the roles of each of the three point mutations contained in Gap71 individually. These experiments have revealed that serine 22 is a site of phosphorylation in the Gal4 DBD and that lysine 23 is essential for S22 phosphorylation, possibly acting as part of the kinase recognition site. Mutation of either residue blocks Gal4 DBD phosphorylation, its subsequent ubiquitylation and compromises the ability of the activator to bind promoter DNA in vivo. These data represent the first report of an essential phosphorylation event that is critical for the activity of this paradigmatic transcription factor.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...