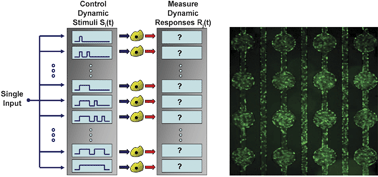
The temporal pattern of a biological stimulus is an important determinant of the resulting cellular response. We present a microfluidic parallel perfusion culture system for controlling the dynamics of soluble cell microenvironments while simultaneously performing live-cell imaging of cellular responses. A “Flow-encoded Switching” (FES) design strategy is developed to simultaneously deliver many different temporal profiles of stimuli, including pulse train widths, lengths, and frequencies, to downstream adherent cells using a single input control. The design strategy uses principles of laminar flow and diffusion-limited mixing to encode the state of the network (the instantaneous stimulus concentrations in each channel) into the ratio of two flow rates, which is controlled by a single differential pressure. To demonstrate the utility of this experimental system, we investigated the effect of dynamic stimuli on NFκB transcriptional activation and cell fate determination. Our results illustrate that transcriptional responses and cell fate decisions depend both quantitatively and qualitatively on the timing of the stimulus. In summary, by encoding dynamic stimuli in a single input pressure, microfluidic flow-encoded switching offers a scalable experimental method for systematically probing the functional significance of temporally patterned cellular environments.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...