LA-ICP-MS studies of zinc exchange by copper in bovine serum albumin using an isotopic enriched copper tracer†
Abstract
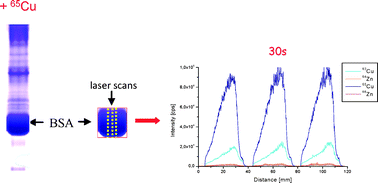
In the present work the binding of Cu and Zn on bovine serum
- This article is part of the themed collection: 2007 International Symposium on Metallomics, Nagoya, Japan

 Please wait while we load your content...
Please wait while we load your content...