A green, fully enzymatic procedure for amine resolution, using a lipase and a penicillin Gacylase†
Abstract
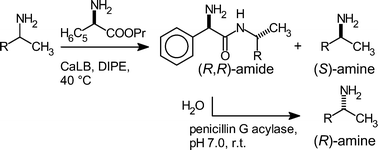
A green procedure for the kinetic resolution of chiral amines via enzymatic
- This article is part of the themed collections: Green and sustainable chemistry and Collection Honouring Roger Sheldon

 Please wait while we load your content...
Please wait while we load your content...