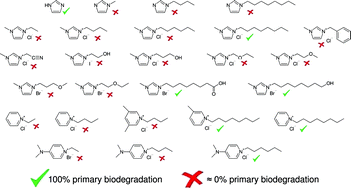
We investigated the primary biodegradation of different N-imidazoles, imidazolium, pyridinium and 4-(dimethylamino)pyridinium compounds substituted with various alkyl side chains and their analogues containing functional groups principally based on OECD guideline 301 D. For the experiments we used two different types of inocula, a freeze-dried mix of bacteria and activated sludge microorganisms from a wastewater treatment plant. The aim of this study was to improve the knowledge base for the structural design of ionic liquids with respect to an increased biodegradability combined with a reduced (eco)toxicological hazard potential. We found a significant primary biodegradation for (eco)toxicologically unfavourable compounds carrying long alkyl side chains (C6 and C8). In contrast for (eco)toxicologically more recommendable imidazolium ionic liquids with short alkyl (⩽C6) and short functionalised side chains, no biological degradation could be found. The introduction of different functional groups into the side chain moiety thus offering a higher chemical reactivity did not lead to the expected improvement of the biological degradation. After an incubation period of 24 days for the 1-methyl-3-octylimidazolium cation we identified different biological transformation products carrying hydroxyl, carbonyl and carboxyl groups. Furthermore, shortened side chain moieties were identified indicating the degradation of the octyl side chain via β-oxidation. Moreover, we propose an electrochemical wastewater treatment as part of an alternative disposal strategy for non-biodegradable ionic liquids. We show for the first time that the 1-butyl-3-methylimidazolium cation was completely destroyed within four hours using an electrolysis double-cell (volume = 1.2 L) equipped with electrodes made of iridium oxide (anode), stainless steel (cathode), and a boron-doped diamond-coated bipolar electrode. The products formed electrochemically were easily accessible to biological degradation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...