The catalytic activity for the production of biodiesel with three morphologically different nanocrystalline MgO materials prepared using simple, green and reproducible methods was investigated. The nanocrystalline samples studied were MgO(111) nanosheets (MgO (I)), conventionally prepared MgO (MgO (II)) and aerogel prepared MgO (MgO (III)). The methods to produce the catalysts included: (a) 4-methoxy-benzyl alcohol templated sol-gel process followed by supercritical drying and calcination in air at 773 K (MgO (I)), (b) from a commercial MgO that was boiled in water, followed by drying at 393 K, and dehydration under vacuum at 773 K (MgO (II)), and (c) viahydrolysis of Mg(OCH3)2 in a methanol–toluene mixture, followed by supercritical solvent removal with the formation of a Mg(OH)2 aerogel that was dehydrated under vacuum at 773 K (MgO (III)). These catalysts were characterized by TEM, DRIFT, and DR-UV-Vis and tested in the transesterification of sunflower and rapeseed vegetable oils at low temperatures, under different experimental conditions: autoclave, microwave and ultrasound. Working with these materials under microwave conditions provided higher conversions and selectivities to methylesters compared to autoclave or ultrasound conditions. Under ultrasound, a leaching of the magnesium has been evidenced as a direct consequence of a saponification reaction. These systems also allow working with much lower ratios of methanol to vegetable oil than reported in the literature for other heterogeneous systems. The activation temperature providing the most active catalysts was found to vary depending on the exposed facet: for MgO(111) structures (i.e.MgO (I)) this was 773 K, while for MgO (110) and (100) (i.e.MgO (II) and MgO (III)) this was 583 K.
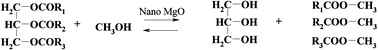
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
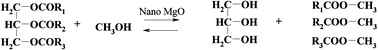

 Please wait while we load your content...
Please wait while we load your content...