Formation of stoichiometrically 18O-labelled oxygen from the oxidation of 18O-enriched water mediated by a dinuclear manganese complex—a mass spectrometry and EPR study†
Abstract
Oxygen formation was detected for the oxidations of various multinuclear manganese complexes by
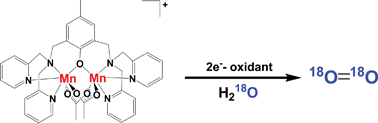

 Please wait while we load your content...
Please wait while we load your content...